Publish Date (HKT) 2022-01-03
[FALSE] Do governments’ COVID-19 vaccination measures violate the Nuremberg Code?

Screenshot of the Facebook post.
The Claim and Our Verdict
- The claim: A Facebook post published Dec. 2, 2021, claims governments’ vaccination measures violate Article 6, Section 3 of the Nuremberg Code, which stipulates that no government can mandate or force medical treatment without individual consent.
- Fact-checking:
- The Nuremberg Code contains 10 principles and is not segmented into articles or sections. So “Article 6, Section 3” proclaimed in the Facebook post doesn’t exist in the Code. The Nuremberg Code does require that “human participants in experiments should give informed consent.”
- Calvin Ho, associate professor at the Centre for Medical Ethics and Law, the University of Hong Kong, replied to our enquiry stating that the clinical trial context should not be confused with mandatory vaccination, which is a public health measure. As a public health measure, the Nuremberg Code will not be applicable in considering whether a vaccination program should be made mandatory.
- Currently, Hong Kong’s COVID-19 vaccination program offers the Sinovac and Pfizer vaccines. The government will inform citizens of possible side effects before vaccination, and jabs will only be administered upon every citizen’s informed consent. According to relevant research, the clinical trials of the Sinovac and Pfizer vaccines have all received the informed consent of the participants in advance.
- Our ruling: Therefore, we rate the claim as FALSE.
News Brief
A Facebook post published Dec. 2, 2021, claims the governments’ COVID-19 vaccination measures violate Article 6, Section 3 of the Nuremberg Code, which stipulates that no government can mandate or force medical treatment without individual consent. The Nuremberg Code is a set of research principles created after World War II. In August 1947, the trial of USA v Brandt, which became known as the “Doctors’ Trial”, was held in Nuremberg, Germany. In the trial, the judges delivered their verdict against Karl Brandt and 22 other Nazi physicians for their inhumane and unethical human experiments in concentration camps. The Nuremberg Code is one result of the Nuremberg trials. Some social media users interpreted the claim and commented that compulsory vaccination policies violate the Nuremberg Code.
As of the issuance of this report, the post had been shared 158 times, and had received 28 comments and 549 likes or reactions.
Fact-checking
According to an article published in The New England Journal of Medicine, the Nuremberg Code contains 10 principles and is not segmented into articles or sections. So “Article 6, Section 3” as proclaimed in the Facebook post doesn’t exist in the Code. The third principle reads, “The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results will justify the performance of the experiment; The sixth principle stipulates, “The degree of risk to be taken should never exceed that determined by the humanitarian importance of the problem to be solved by the experiment.” Both points are not related to “involuntary treatment” purported in the claim.
The first principle, the only one relevant to the claim, reads, “the voluntary consent of the human subject is absolutely essential.” It indicates that the experimental subject should have legal capacity to give consent and should be made known the nature, hazards and effects upon his health or person which may possibly come from his participation in the experiment.
“The duration of a clinical trial is essentially a scientific question, although the commencement, modification and termination of a clinical trial are subject to ethical and regulatory oversight. Ethical and regulatory oversight ensures that the principles of the Nuremberg Code are observed where trial participants are concerned. The clinical trial context should not be confused with mandatory vaccination, which is a public health measure that is only considered after the drug regulatory agency in the jurisdiction concerned has granted approval (on emergency use basis or otherwise) for the vaccine to be used outside of a clinical trial setting. The drug regulatory agency will only grant approval after there is enough evidence to show that the vaccine is generally safe and effective. As a public health measure, the Nuremberg Code will not be applicable in considering whether a vaccination programme should be made mandatory,” Dr. Calvin Ho, associate professor at the Centre for Medical Ethics and Law, the University of Hong Kong, told HKBU Fact Check.
The “involuntary treatment” purported in the claim, or the mandatory vaccination policies commented by social media users, therefore, does not belong to clinical trials and the Nuremberg Code also does not apply to them.
Taking the two vaccines offered in Hong Kong as an example, people will be asked to sign or agree to the informed consent form which will include the possible adverse events in receiving COVID-19 vaccination. Under Section 8, Prevention and Control of Disease (Use of Vaccines) Regulation made by HKSAR, authorized vaccines need to be administered with informed consent.
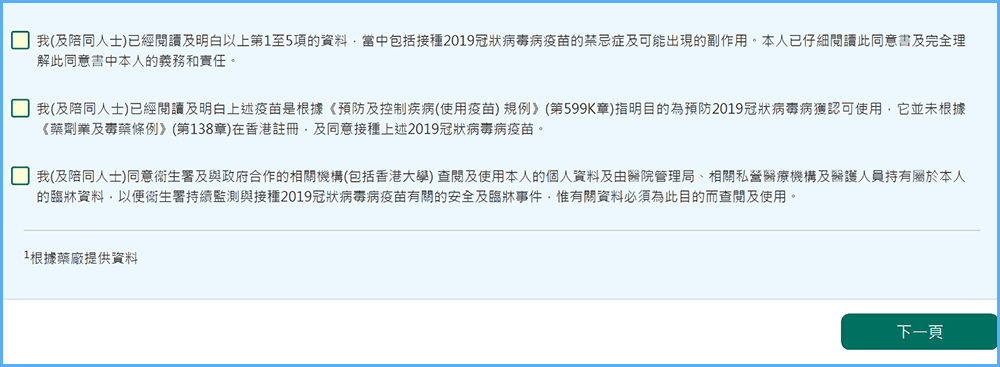
Screenshot of the informed consent form on the website of Hong Kong’s COVID-19 vaccination program.
An article published in the journal of the Lancet Infectious Diseases claims that written informed consent was obtained from each participant during the phase 1/2 clinical trials of the Sinovac vaccine. The clinical trial protocol and informed consent form were approved by the local ethics committee. Another article published in the journal, Trials, shows that the safety and efficacy study of the Sinovac vaccine in Brazil was reviewed and approved by the Brazilian National Ethics Council—CONEP. In addition, the local ethics committee of each clinical research site has reviewed and approved the protocol. All study participants in the trial are adults and provide informed consent before entry in the study.
An article published in The New England Journal of Medicine also shows all participants involved in the Pfizer vaccine’s phase 2/3 trials provided their informed consent.
In conclusion, the Sinovac and Pfizer vaccines’ clinical trials both obtained experimental participants’ informed consent. The clinical trials of the vaccines do not violate the Nuremberg Code. The public have also been informed of potential risks before vaccination and agreed to the informed consent form. As a public health measure, the Nuremberg Code will not be applicable in considering whether a vaccination programme should be made mandatory.
Conclusion
Therefore, we rate the claim as FALSE.
References
- Facebook post, Dec. 2, 2021.
- The New England Journal of Medicine, “Fifty Years Later: The Significance of the Nuremberg Code,” Nov. 13, 1997.
- The University of Hong Kong, Centre for Medical Ethics and Law, Calvin Wai-Loon HO.
- Hong Kong government, COVID-19 Vaccination Programme—Booking System.
- Hong Kong government, “Prevention and Control of Disease (Use of Vaccines) Regulation,” Dec. 24, 2020.
- Lancet Infectious Diseases, “Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial,” Nov. 17. 2020.
- Trials, “Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac – PROFISCOV: A structured summary of a study protocol for a randomised controlled trial,” October 15, 2021.
- The New England Journal of Medicine, “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine,” Dec. 31, 2020












 :
: