Publish Date (HKT) 2020-08-27
[PARTIALLY TRUE] Does spinal muscular atrophy treatment with nusinersen cost over 3,000 times more in China than in Australia?
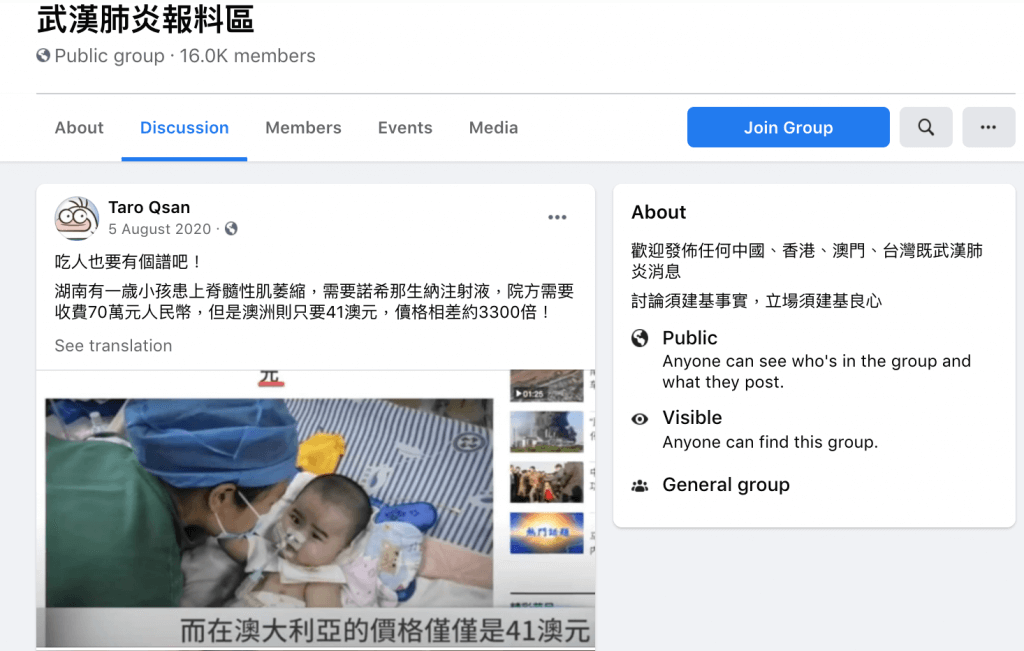
A screenshot of the Facebook post
Outline
- A Facebook post claims that nusinersen, marketed as Spinraza, a prescription medication used in treating spinal muscular atrophy (SMA), costs more than 3,000 times more in mainland China than in Australia.
- As the Australian Government’s Pharmaceutical Benefits Scheme (PBS) subsidies, eligible patients pay only AU$41 (approximately HK$230) per dose for nusinersen (12 mg/5 mL injection). Without the subsidy, the drug’s retail price reaches AU$125,000 (approximately HK$697,500) per injection. In mainland China, a dose of the same injection costs ¥699,700 (approximately HK$782,800) as nusinersen is not yet included in the national medical insurance catalogue of China.
- With the Australian Government’s subsidy, the price difference of the drug between the two countries is as high as 3407.39 times, while it is only 1.12 times when comparing the retail prices of both countries without the subsidy of the Australian Government.
- The claim that nusinersen costs over 3,000 times more for SMA patients in mainland China than those in Australia is PARTIALLY TRUE as it compares the retail price in China with the government-subsidised price of Australia.
News Brief
A young father in Hunan province, China, asked for crowdfunding to cover medical expenses of his son, who suffered from the rare genetic disease SMA. The boy was fighting for his life at a hospital’s intensive care unit and his dad could not afford the specific medication of nusinersen at a cost of ¥0.7 million (approximately HK$0.78 million) needed to treat SMA. Shortly after the post was published, content tagged with “desperate parents need to pay ¥0.7mil per dose of medication for their son” occupied the hot search list of the Chinese microblogging website Sina Weibo. As of the issuance of this report, the topic has attracted more than 90 million views.
A screenshot of the hot search list of Weibo
On Aug. 5, 2020, a netizen named “Taro Qsan” posted two screenshots of relevant news reports to a public group on Facebook with the caption, “That’s totally out of line! A one-year-old Hunan child suffering from SMA needs nusinersen injections and each injection costs ¥0.7 million. The same drug only costs AU$41 in Australia. A price difference of almost 3,300 times between the two countries!”
According to the website of the Department of Pediatrics and Adolescent Medicine of the University of Hong Kong, SMA affects 1/6000 to 1/10,000 live births and is a leading genetic cause of death in infant. It encompasses a group of inherited neuromuscular disorders which affect the nerve cells, called motor neurons, in the anterior horn cells of the spinal cord that control voluntary muscle movement. In December 2016, the US Food and Drug Administration (FDA) approved nusinersen as the first approved drug to treat SMA.
Fact-Checking
The claim questions: Is it true that “SMA treatment with nusinersen costs 3,000 times more in China than in Australia”?
The netizen “Taro Qsan” posted two screenshots of relevant news reports on Facebook. One was captured from a news video, which showed that the Hunan baby suffering from SMA could not receive a nusinersen injection because his parents could not afford it. Another picture showed a bottle of nusinersen for injection.
BU Fact-check Team used Jeffrey’s Image Metadata Viewer, a reverse image search engine, and found that one of the images uploaded by the Facebook netizen first appeared in a news report published by the New Tang Dynasty television on its website on Aug. 3, 2020, at 10:13 a.m., Beijing Time. The news report was entitled “Lifesaving drug for Hunan infant costs ¥0.7 million per dose. Chinese netizens: the same only costs AU$41 in Australia.” The reverse image search results showed that the image was posted on Aug. 3, 2020, at 5:57, but the time zone was unknown. Considering that the two publication times were so close, it was inferred that the Facebook image was a screen capture from the New Tang Dynasty television’s website.
A comparison between the reverse image searching result and the image shown on the website of New Tang Dynasty television
According to our findings, the Australian Government subsidises treatment with nusinersen. Greg Hunt, Australia’s Minister for Health, announced in a press release dated May 6, 2018 that starting from Jun. 1, 2018, PBS would subsidise treatment with nusinersen (marketed as Spinraza) for curing patients under the age of 18 years with SMA types 1, 2 or 3a.
As shown on the website of PBS, the general patient charge for a dose of nusinersen (12 mg/5 mL injection) is AU$41 (approx. HK$230) while the dispensed price for maximum quantity (DPMQ, i.e., the pharmacy selling price plus dispensing fees) per injection is AU$110,000 (approx. HK$613,800).
However, PBS’s subsidy does not cover nusinersen treatment for SMA type 3b patients or those above the age of 18 years, so they need to pay the full price. According to a news report published by Herald Sun on Aug. 30, 2018, SMA patients of 18 years and older had to pay AU$125,000 (approx. HK$697,500) per injection. As reported by Stuff, the top news site in New Zealand, on Mar. 12, 2019, Julie Cini, the Chief Executive of the non-profit organisation SMA Australia, said the retail price for each injection in Australia was AU$125,000.
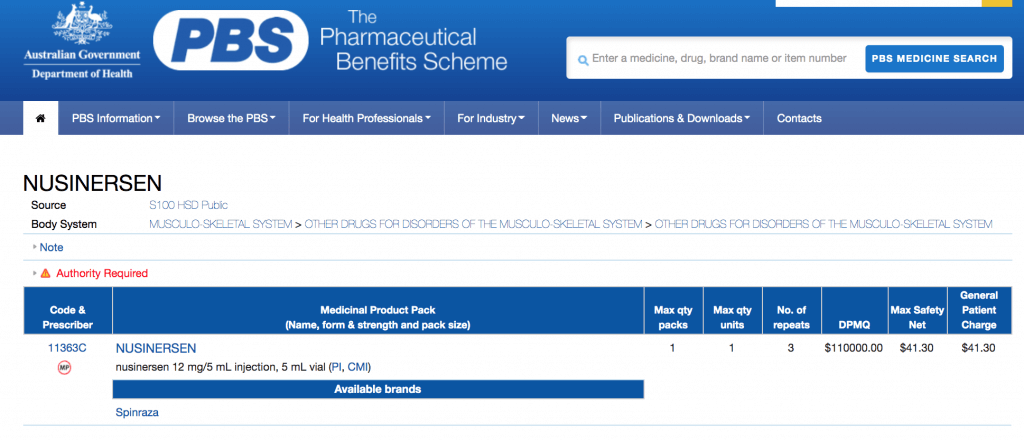
A screenshot of the price of nusinersen on the website of PBS
On Aug. 6, 2020, XinhuaNet, the official state-run press website in China published a news article titled “¥700,000 per injection, very much expensive than abroad?” It was reported that since 2019, nusinersen had been approved to be imported to mainland China with a selling price at ¥699,700 (approx. HK$783,700) per dose. The treatment was not covered by the medical insurance and therefore, SMA patients in China needed to pay out of pocket.
Biogen, a biotechnology company in China, posted an article entitled “Biogen’s Statement” with its official WeChat account on Aug. 6, 2020, stating that nusinersen was a self-financed drug in mainland China. The price of the drug was obviously different from the out-of-pocket cost a patient should pay with a healthcare subsidy. Biogen pointed out that, as at Jun. 30, 2020, treatment with nusinersen had been approved in 50 countries/regions and subsidised by 40 countries/regions, including Australia.
A screenshot of Biogen’s official statement regarding the price of nusinersen
In accordance with the above information, nusinersen is covered under the Australian Government’s Pharmaceutical Benefits Scheme. In Australia, patients under the age of 18 years with SMA types 1, 2 or 3a only pay HK$230 per injection while other SMA patients have to pay around HK$697,500. In mainland China, as the drug is not covered by the medical insurance, patients have to pay the full price HK$783,700 out of pocket.
In summary, taking into account of the subsidy provided by the Australian Government, the price of a dose of nusinersen in mainland China is 3407.39 times higher than that of Australia (HK$783,700 vs. HK$230). Otherwise, the price difference is 1.12 times only (HK$783,700 vs. HK$697,500).
Conclusion
In conclusion, the claim that treatment with nusinersen costs over 3,000 times more in mainland China than in Australia is partially true as the comparison is simply made between the retail price in China and the cost under the subsidy by the Australian Government. After reviewing relevant news reports and information and comparing the healthcare policies of the two countries, it is concluded that the claim is PARTIALLY TRUE.
References
- The post in a public Facebook group
- Content tagged with “desperate parents taking about treatment that costs ¥0.7M per dose for their son”, occupying the hot search list of Sina Weibo.
- Website of the Department of Paediatrics and Adolescent Medicine of The University of Hong Kong: 5q spinal muscular atrophy
- Report published by the New Tang Dynasty television in its website, entitled “Lifesaving drug for Hunan infant costs ¥0.7M per dose. Chinese netizens: the same only costs AU$41 in Australia”.
- Press release by Australia’s Minister for Health
- Website of the Australia Goverenment’s Pharmaceutical Benefits Scheme – information about nusinersen
- Report published by Herald Sun on Aug. 30, 2018: “Life-changing drug Spinraza for spinal muscular atrophy patients costing adults at least $500,000”
- Report published by Stuff, a news site in New Zealand, “SMA patients could wait four years for new wonder drug Spinraza”
- Spinal muscular atrophy (SMA) Australia– Non Profit Organisation
- XinhuaNet report: “¥700,000 per injection, very much expensive than abroad? – Focusing on whopping priced drug for a rare disease”
- Biogen Statement published on its official WeChat account












 :
: